always, at the end of every lab:
A. return all equipment to where it was, clean lab bench;
B. put your things (including goggles) in lab drawer, lock it;
C. wash your hands, in lab and in restroom (north of labroom).
also — Assignment 4: Tips and Directions
Here is a repeat from early November.
A Change of Policy: I will not email/text (as I have several times before)
if you haven't done the Prelab Quiz and it's close to the 3:15 deadline.
( Put this on your daily/weekly to-do list, and remind yourself. )
SUGAR — in Soft Drinks and Fruit Juices (Dec 4 or 6)
Sugar Lab — For the Prelab Quiz, this week no special tips
are needed; just read the lab manual, and think carefully.
As usual, the CiC-textbook (and your general knowledge) may be useful,
for example by their definition of solution in glossary, or using the index.
Sugar Lab — Useful Tips for the Green Pages:
• C - Study "Preparing a Calibration Curve" (page 14-4), and in the
prelab talk I'll explain more about the useful over-and-down technique
that is illustrated by dashed lines on the graph (on 14-4); look at
lab-room blackboard to see the "steps" I want you to show on graph.
• 1a-1b: from Exam 3, to help you prepare for the Final Exam and
answer these questions, read Lecture
21 (Oct 29), Slides 13-26.
• 3 - Imagine that you are a scuba diver, and you want to lift a
sunken boat to the surface. You have many plastic bags, tanks of air,
duct tape & rope. How can you "use what you have" to raise the boat?
• 4a - Do this by using Conversion Factors (CFs) in the usual way:
amount (CF)(CF) = amount [with same "size" but in different units] ;
per cent means "per 100" so (for example) if 43% of students are
wearing blue jeans, the CF is (43 students with bj / 100 total students).
• 4d - Imagine dumping this much sugar into a glass of water;
then add some artificial flavors & colors, and drink it! :<(
• E-1a,1b - Does a bowling ball float? Predict, then read this.
• E-3b - Think carefully, asking "what things change as time goes by?"
• E-4 - They expect you to assume shielding is proportional to mass.
• E-5 - Design a very simple experiment to produce data that (along with
"water is 1.00 g/mL") lets you calculate a numerical density for the oil.
Common Plastics – Classification & Identification (Nov 27 or 29)
Plastics Lab — Useful Tips for the Prelab Quiz,
which also will help you on Quiz 8, Exam 4, and the Final Exam.
#1 and #2 - use the handout for "Functional Groups" in Learn@UW.
#3 - Section 90, do after W's lecture about Condensation Polymers;
89, do your best -- I'll post a handout for this tonight,* which will show
"remove HOH and hook the losers together" where "the losers" are the
atoms (O or N, C) that lost H and OH. Also, pages 382-387 of CiC.
And, as always, be sure each atom (C N O ...) has its correct # of bonds,
when monomer-linking bonds "across the bracket" are included. These
require "careful comparing" which requires investing detective-time.
* I won't get the handout finished tonight, but here is a relevant part:
How to Visualize & Draw Repeating Units: Page 387 shows "parts that
leave" (OH, H) in red. Remove these parts (that form HOH) and
what survives (i.e. the non-red parts) are the repeating-unit.
For polyester, use p 384 (imagine the far-left H and far-right OH are red,
as on p 387); or use the molecule in the middle of the top row on 385.
#4 - see Lecture 30 (Nov 26), Slides 11-34; and Big 6 on CiC, pg 378.
#5 - CiC (371-373), Lecture 30 (slides 33-end, especially 63, 70, 89),
and my handout late tonight. (inside-the-brackets always has 4 C's)
Plastics Lab — Useful Tips for the Green Pages:
These require careful thinking, but no special "tips" except...
you may find it useful to use page 378 for "The Big Six" table, to help
you use “chemistry logic” for the functional groups in each polymer.
The Energy Content of Fuels (Nov 13 or 15)
Energy Lab — Useful Tips for the Prelab Quiz:
As usual, just DO IT and you'll get all (or most) of the 10 points.
• 1 - Read CiC, from bottom of 179 ("Although the heats...") to the
middle of 181 ("... their nonoxygenated counterparts."), especially
page 181. For some #1-questions, you'll also need a definition of
"ether" as a general type of organic molecule, which is on 412.
• 2,3,4 - Read lab manual, and consider all options before choosing one.
• 5 – Understand the calculations described on 11-4 & 11-5. If they
specify a precision to use in your answer, do it; if they don't, and you
want full points
you should (based on my experiments in trial quizzes)
ignore sig figs and enter your answer to the nearest whole number.
Sometimes you won't use all information given, and sometimes you will.
Read their "hint" for each problem, and use it to get points on the quiz.
Energy Lab — Useful Tips for the Green Pages:
• 1-2 - A good way to understand the graph-trend in #2 is to think about
"grams of oxygen per gram of fuel" which varies from .35 (for ethanol)
to .12 (for 1-octanol). But use "g of C per g of fuel" for the graph.
• 3a - In the prelab talk, I will explain how you should calculate the slope,
by showing (with circles) the initial & final points of an interval for which
you will
find "(yf - yi) / (xf - xi)" and show your work with this equation.
• 3b - To get "amount of heat given off per gram of carbon" you must
combine the two conversion factors (and corresponding numbers) in
#1 (table) & #2 (graph), in the same way that you do other calculations
that
use conversion factors; to get credit for #3 you must show the
numerical value of "J / g of C" (using class-data) for ethanol, 1-butanol,
and 1-octanol, and then choose-and-explain based on these values.
•4 - Explain how they are similar, how they are different, then predict.
•5a - Answer by describing "what you will hold constant" and "what will
change"
in one or more equations on page 11-4, if you use 200 mL
instead of 100 mL, and then explain why there is no overall change.
• 5b - To imagine "what might happen," answer this for more-extreme
situations with 10 mL and also 1000 mL, instead of 50 mL and 200 mL.
• 6b-6c - Read CiC, from bottom of 179 ("Although the heats...") to
the middle of 181 ("... their nonoxygenated counterparts.").
Chemical Moles: Converting Baking Soda to Salt (Nov 6 or 8)
Below you'll see "a lot" but please don't feel overwhelmed. As with any
complex project, move one step at a time and use all available resources
— these tips, the lab manual, CiC-textbook, and Lectures — to work thru
problems in the Green Pages and then the Prelab Quiz, trying to get all of
these points and (also important) learn everything you can, because this
will help you get points in the future on Quiz 7, Exam 3, and beyond.
A Change of Policy: I will not email/text (as I have several times before)
if you haven't done the Prelab Quiz and it's close to the 3:15 deadline.
( Put this on your daily/weekly to-do list, and remind yourself. )
An Unusual Strategy: This week, I recommend doing both parts
(Prelab Quiz & Green Pages) at the same time, due to the major overlaps
between them. Also use the lecture (M, Nov 5) and CiC-textbook.
Useful Tips for Green Pages — I suggest doing Questions 2-6 before
the Prelab Quiz, but (as always) invest time but don't waste time;
if you "get stuck" you can discuss it in lab with other students, and me.
• 4-5: use the index of CiC to find a key chemical equation using the
most obvious index-word; another index-word is "solubility" which is
a word used in chemistry to describe what does and doesn't dissolve.
• 3 & 6: First do #5, because in all of these reactions (5, 3, 6)
the "active parts" form H2CO3 (with 2 H+ + CO32-, or H+ + HCO3-)
which then continue onward to form "H2O (l) + CO2 (g)", and the
"spectator ions" combine to form a salt, which is a general term for any
ionic +– combination that is neutral, that is not acidic or basic. When
you do the "active parts" correctly, the salt should be formed correctly
so it has zero charge, due to the ion-charges, and numbers of each ion.
• 1: use detective work from "Gases in a Breath" lab, and CSN Grid.
• 2c: This requires a reaction, so do it before your area-cleanup.
Useful Tips for Prelab Quiz: As usual, do it M afternoon, or later.
#1-2: Carefully read the directions about required answer-formats.
Do #1 after you have mastered Questions 3-6 on the Green Pages.
For #2, study their examples for zeros in significant figures, plus
Slides 19-20 (Lecture 24, M, Nov 5), and (as suggested in my email last
night) "notice where the zeros are, relative to the decimal point, and
relative to the non-zero numbers" and also (in Slide 20) the important
difference between addition/subtraction (think "decimal places") and
multiplication/division (think "sig figs"). For the actual problem in #2,
study Slides 18 & 21-22 plus 32 and "notice the canceling of units"
as in “2 pounds of apples (90 cents / pound of apples) = 180 cents".
#3-4: Use your lab manual, read-and-think carefully.
#5: Use the same logic-and-math that you use to know that
2 million cars, each with 4 tires, have 8 million tires; analogies are that
like a million, a mole is just a number; and it can be equally easy to
visualize-and-imagine (4 tires/car) and (2 chloride ions / SrCl2);
and for both problems, use "canceling of units" in the math-conversions.
pH of Rain & Other Common Substances (Oct 30 or Nov 1)
As usual, do the prelab quiz after lecture M (89) or W (90). You should
read in CiC, Sections 5.5 & 6.1-6.4 (it's well written, and you'll want to
study it for Exam 3 anyway) and parts of 6.6 (the start is interesting for
labwork, and the ending for chemistry of SOx and NOx). For prelab quiz,
my main tip is DO IT so you don't give away an easy 8-10 points; also,
• #1-2 (use M lecture, CiC 6.4), • 3-4 (M lecture, CiC 6.1-6.3, 6.6),
•5 (CiC 6.3, and if [H+] = 2.57 x 10-5, [OH-] = 3.89 x 10-10, ph = 4.59).
For the lab and Green Pages, basically just "know your stuff"
by reading the lab manual, and learning from lectures & CiC, plus:
• Questions 1-2 (you should know these from your prep for Exam 2),
• 3-4 (use index of CiC), • 5 (do before lab, or during lab on a
computer in Chem 1375, or your own laptop if you bring it).
suggestions for "Refrigerant Gases" (Oct 23 or 25):
As explained in my email with your score, last week's lab was unique
(to help each of you begin to master essential
ideas-and-skills for 108),
and this week we'll
return to regular grading, as with the first 5 labs.
• This week, most of what you need is in the lab manual. For the
prelab quiz, all you need to know for #1 is explained within #1, for #2
use the two far-left columns in my CFC-Grid. For #3-4-5, use info
from the lab manual, plus logic. / For the Green Pages, some info
from the Oct 22 lecture can be useful, but mainly you'll use info from
the lab manual, plus your own observations, logic, and thinking.
suggestions for "Molecular Models" (Oct 16 or 18):
I won't say much because this lab is mostly self-contained, with what
you need supplied in the lab manual and the computer program, plus
lectures today (M, Oct 15) and (although you don't need it for lab) W.
Also, for this lab & throughout Oct/Nov/Dec, the bottom of my latest
worksheet (W, Oct 10) will be helpful, re: how many bonds each atom
"wants" when it's neutral or charged, and (especially for the prelab quiz)
the importance of unshared/nonbonding electrons for molecular shape.
Also, you won't do Part D this week, although it will be important later.
suggestions for "Light in Our Atmosphere" (Oct 9 or 11):
Prelab Quiz — As usual, read-and-think logically; #3 requires careful
thinking, but you can figure it out by using logic (usually 4 of the 5 options
are scientifically foolish) and learning from the Introduction (lab manual,
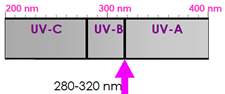
(page 6-1) and from this description and the picture here.
Almost all of the lab-Questions are about ideas you'll need to learn for
Exam 2, and you can learn a lot by yourself when you carefully read:
• The Introduction (p 6-1); all of the info is useful, although you
won't need to do the logarithm-math; but the concept of a logarithmic
scale (as in the example of a 10-fold increase in going from 1 to 2) can
be useful in many areas of life, including (later, for pH) Chem 108.
• Parts A-E, which parallel the Questions in Parts A-E.
In your CiC-textbook, carefully study (i.e. think logically about) pages
• 77 – Figure 2.7 and the first sentence ("The... radio waves.")
let you answer key questions for Exam 2: What is the relationship
between wavelength and energy? Is it direct or inverse? (i.e.,
as wavelength increases, does energy increase or decrease?)
• 110 – As with many things in life, with greenhouse warming
some is good but too much (when the normal is "enhanced") isn't.
• 80 – For the UV-absorbing reactions of O2and O3, and
Table 2.4 which is illustrated in a lecture-slide from Fall 2011,

suggestions for "Light in Our Atmosphere" (Oct 9 or 11):
As with every lab, the introduction (page 5-1) has useful information,
and so does Table 1.1 (pg 20 of CiC), as explained in lecture Monday, Oct 1.
For the Questions (on pages 5-9 and 5-10):
#1 (you can do it before lab, and it's good practice for Exam 1);
#2 (pg 245 of CiC);
#4 (pgs 249-250, CiC);
#5 (designing experiments is a useful skill to develop,
so invest time to think about this, but don't waste time).
LAB SCORES (on Green Pages and in Learn@UW)
Due to Excel-based processing (with a multiplier for each section),
each red "-1" that is marked on a Green Page deducts less than this
(typically it's -.25 to -.75 points) from your score in Learn@UW.