This web-page is a rough outline of
a paper I'll submit to The Journal of Chemical Education in the near future. [update: I did not submit it to JCE in 1999.]
The introduction to my paper will cite two papers from J Chem Ed:
one is by Stephen J. Hawkes (JCE, 1992, pp 542-543), about the mechanism
for acid-base reactions; another (maybe by Miller in JCE, 1992; I'll
check this later) discusses the use of anthropomorphic analogies to help
students form vivid mental images of chemical phenomena.
Hawkes explains why common methods of
teaching can "create a false concept of the action of an acid, as if
it somehow expelled its proton by means of some internal force," and
why a more accurate description is that "a base must tear the H+ from the attractive forces holding it to an acid, breaking its bonding by
superior force."
I agree, and for two decades I've been
using the conceptual analogies of proton warring and proton wooing for the purpose of helping
students to construct vivid mental images of atomic-level events involving
protons and electrons within molecules, and to connect these images with
symbolic representations of the atomic-level events in chemical equations
comment: In the manuscript I'll
submit to JCE, the introduction (and the rest of the paper) will be developed
in more detail. In this draft I'll just outline the basic themes.
Fundamentals
As in other chemical reactions,
an essential driving force for acid-base reactions is the attraction between
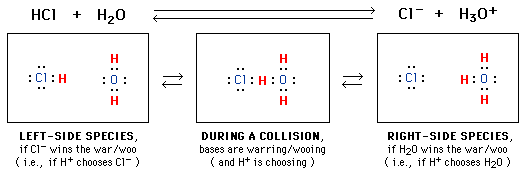
positive and negative charges. As shown in the center picture below,
during a collision one proton (H) is simultaneously
between two electron pairs (:), one on Cl and another
on HOH. Because each electron pair has negative charge,
each attracts (and is attracted to) the positively charged proton, and each
would like to be near this proton (in the form of a covalent bond involving
the proton) after the collision.

Two possible outcomes are shown.
When the electron-pair on Cl- wins the battle, we get the species
on the left side of the equilibrium equation, HCl + H2O.
But when H2O wins the war, we get the right-side species, Cl-
+ H3O+. The left and right sides of the equation
correspond to victories by Cl- and H2O, respectively.
This diagram above can help students to
understand the fundamentals of reactions, and to interconvert between different descriptive representations (in chemical equations,
electron-dot pictures, verbal
descriptions, and mental representations)
of an acid-base reaction.
Two Perspectives – Warring
and Wooing
We can think of the collision-interactions
in two ways.
Proton Wars:
Every collision is analogous to a tug-of-war between two bases, and the stronger
base (it's the base that is pulling more strongly) takes the proton.
Proton Woos:
Every collision is analogous to a competitive courtship. During the
collision both electrons make themselves available to the proton, and
the proton chooses to depart with the electron-pair that is more attractive,
when all factors are considered.
[update in 2010: Both perspectives, warring and wooing, are technically equivalent but are mentally different, leading to different mental models. Both can be useful ways to think about the +- interactions (attractions & repulsions) that are key concepts in modern chemistry. Thinking about the collision as a WAR adopts the perspective of a proton being the passive “reward” won by the stronger competitor, while with a WOO the proton is an active “chooser” of its partner.]
In either perspective, • a strong acid is a strongly reacting acid that loses most of its protons, so it can produce a strongly acidic solution (if the acid's concentration is high enough), and • the strongly acidic HCl does not “want” to donate a proton,
but its conjugate base (Cl-) is not strong enough to hold onto the proton during most collisions of HCl with HOH.
By contrast with a misleading equation
(HCl ➞ H+ + Cl-) that implies action by the acid,
the representations above — the Bronsted-Lowry equation (HCl + H2O
⇄ H3O+ + Cl-) and the electron-dot
pictures — place the focus where it should be, on the bases and
(more fundamentally) the base's electrons as these interact with
a proton.
SYMBOLISM: The meaning of key symbols (H and H+) varies with context. In a chemical formula (and thus a chemical equation) or in a sentence, H is a neutral H-atom (a proton with an electron); but in an electron-dot picture, H is a positively charged proton. In a formula, equation, or sentence, a proton is H+; but a proton is H in the electron-dot picture of a multi-atom molecule.
SIMPLIFICATION: I've
treated acid-base reactivity as a purely covalent process, ignoring the
effects of solvation (and complex species such as H4O9+)
and entropy. { How significant are solvation effects and (especially)
entropy considerations? To what extent is it true that the “stronger”
and “more attractive” electron pairs will go away with the proton,
especially when the solvent is a competitor? If we consider only covalent
electrical forces, the reaction “HCl (aq) --> H+ + Cl-”
is ludicrous, but so is the dissolving of NaCl, which does occur.
{ I'll visit the library tonight to get tables of delta-H and delta-S, but
so far I haven't tried to estimate these contributions. }
In the JCE paper I'll probably keep it simple by briefly mentioning these
factors as outlined in the following paragraph, plus a clarifying endnote,
and citing papers with a more technical treatment. } /
Also, references to simple "electron pairs" ignores the complex
spatial distributions and dynamics of electrons.
An educationally useful approach, especially
at the level of general chemistry, is analogous to a strategy used in physics
--- thinking in terms of a simplified system plus correction factors. For example,
in physics we can first calculate the motion of a hockey puck simplistically
by ignoring friction and air resistance; then we add correction
factors for the effects of friction and air, and notice the difference
this makes in our predictions, compare both sets of predictions (using the
simplified and modified theories) with observations, and learn. /
For an acid-base reaction, a covalent proton war
is a simplified starting point that can be modified by considering other
factors, especially solvation and entropy.
A corrective modification might occur by considering the ways in which solvation
affects the characteristics of each electron pair involved in the proton
war, when we are thinking about "the factors affecting the strength
of a base" as discussed below.
THREE EXTENSIONS follow: 1) factors
affecting basicity/acidity, 2) equilibrium, and 3) an analogous
analogy (from proton wars to electron wars).
FACTORS AFFECTING THE STRENGTH
OF A BASE
An interesting follow-up question
for students is: What makes one electron-pair a stronger warrior,
or a more appealing suitor, compared with another electron pair? Suitable
Aesop's Examples, each chosen to illustrate a specific concept, include:
Comparing the basicity of NH2-
and NH3. If all other factors are equal, in a "conjugate
series" the more negative a molecule containing an electron pair, the
more negative this electron pair will be, and the stronger it will be as
a warrior and wooer, so NH2- (when it grabs a proton
to become NH3) is a stronger base than NH3 (when it
becomes NH4+).
The acidity of CH3CH2OH
vs CH3COOH, of alcohol vs carboxylic acid: Comparing the
conjugate bases for each, in CH3CH2O- the
O has a full negative charge, but in CH3COO- there
is resonance so each O has only a half negative
charge, thereby making the electron pairs on these Os much weaker as a warrior
or wooer, so acetate is a weaker base. { And its conjugate, acetic
acid, is a stronger acid, compared with the corresponding alcohol. }
/ After developing the concept of resonance and its effects
on the electron pairs, we can ask students, "If you were a positive
proton, which would you prefer being close to, a half-negative charge or
a whole-negative charge?"
CH3COOH vs CF3COOH:
Because F is highly electronegative, compared with H, the Fs will pull electrons
away from the COOH end of the molecule, thereby making these O's less negative
and less strong as a warrior/wooer, so CF3COO- is
a weaker base because it isn't as strong at grabbing protons, and CF3COOH
is a more strongly reacting acid because it isn't as strong when it tries
to hang onto its protons.
Other examples (such as HF vs HCl, alkenes
vs alkynes,...) can also be used, depending on students' experience.
REALITY CHECKS: For each example
we can tell students that our chemical intuition, based on a logical analysis
of positive-negative attractions, is supported by experimental observations.
{ And if our chemical intuitions ever fail, this can be especially interesting!
}
DYNAMIC EQUILIBRIUM:
The type of collision shown above (plus spatial variations) is happening
in every part of a solution, over and over, many billions of times each
second. In another paper (unpublished), which outlines an intuitive
approach to the quantitative aspects of acid-base equilibria, I ask students
to do a thought experiment by imagining a "magic
camera" that lets them "see a snapshot" for a typical outcome
(defined by what we observe in physical experiments)
of these billions of battles, captured at an instant of time. /
Then we can gather the results from many physical experiments with different
acids and bases, and summarize our observations in a table that shows the
relative strengths of acids and of their conjugate bases.
ELECTRON WARS (and WOOS)
The same type of anthropomorphic
analogy that is used for acid-base reactions
can help students visualize oxidation-reduction reactions
as a battle for electrons (in an electron war)
and as a competitive courtship (in an electron woo).
/ And a similar qualitative treatment of empirical data, using
tables to show the oxidizers and reducers (or the acids and bases) that
are strong and weak, can be done with either electron wars or proton wars.