Learning from Experience:
An "Aesop's Activities" Approach
to Higher-Level Thinking Skills
in the General Chemistry Lab
a revised version of this
page
(split into "discussion based labs"
and "Aesop's Activities") is in
a
new location
by Craig Rusbult, Ph.D.
This website outlines a proposal for
using undergraduate general chemistry labs
to help students learn higher-level thinking skills.
1. What is an Aesop's Activities approach?
Aesop's Fables are designed to teach lessons about life.
By analogy, Aesop's Activities can teach
the higher-level thinking skills used in science.
Teachers should provide opportunities for experience,
and also help students learn more from their experience.
An Aesop's Approach to developing instruction,
motivated and guided by a general objective of
helping students learn how to think more effectively,
involves a goal-directed coordination
of activities and methods:
1) define goals for education in terms of the skills
(and associated concepts) to be learned by students,
2) design activities that provide experience with these skills,
3) develop methods of teaching that help students learn more
by directing attention to "what can be learned" from their experience.
Educators should make decisions based on merit, not tradition, by examining every activity (old or new) and asking whether it performs a useful educational function. But this radical attitude should be tempered by a recognition that -- when our objective is to achieve maximally beneficial results in a limited amount of time -- instead of aiming for a fresh beginning with a new set of goals, activities, and methods, often it is more practical and immediately productive to build on what already exists. This approach, with a flexible overlapping of steps, begins with a goal-oriented analysis of activities now being used in labs: a careful examination of these activities (in Step 2) stimulates productive thinking about goals (in Step 1), which inspires revisions or supplements to existing activities (in Step 2). Step 3 is a logical extension of this analysis: we just add reflection activities -- which encourage students to think about what is being done (Step 2) and what can be learned (Step 1) -- to the activities already being done in a lab.
{ comments: I've had plenty of experience with Step 3 of this strategy
for "building on what exists" while working as a Teaching Assistant
in physics and chemistry, as I diligently searched the existing labs (selected
by the department) for learning opportunities and for methods of directing
students' attention to these opportunities. / A "building"
approach is consistent with two practical principles for making educational
reforms more appealing for widespread adoption: the proposed reforms
should be immediately productive, without requiring a long period of "delayed
reoptimization" in which the teaching quality is temporarily lower
than it was before the reform, and without requiring a large investment
of development time (for adjusting the reforms to fit the local situation)
or preparation time for teachers (so they can learn how to use the new methods
effectively). }
comment: Most of the ideas I'll be describing will look familiar, due to a convergent agreement among many educators (including myself) about goals and methods for instruction, and because I have borrowed from and have been inspired by the work of others. But I think you will also find some new ideas and fresh perspectives that will contribute "added value" to the educational community.
2B.
Discussion-Based Labs
Discussion-based
teaching (DB) provides an organizing structure that promotes interactions and learning.
To prepare for a DB lab, I split a lab into parts and develop mini-activities
(observations, data analysis, questions,...) for each part. When a
group is ready to discuss Part 1, they call me over; when everyone
is satisfied that our discussion is over, the students move on to Part 2,
and I make an X in the appropriate cell of a discussion grid:
Student Groups |
Lab |
A |
B |
C |
D |
E |
F |
G |
Part 1 |
X |
||||||
Part 2 |
|||||||
Part 3 |
|||||||
Part 4 |
When a group has X's for all parts, they're
free to leave. During a lab the teacher must improvise in an effort
to achieve optimal pacing; sometimes, so students don't have to wait
for the teacher, two or more groups combine for a discussion. { But
if students must wait, they can talk with each other or begin work on the
next part of the lab. }
Most students enjoy DB labs because, compared with traditional
labs in which they write reports and get feedback that is delayed and minimal,
with DB the students have more learning and more fun, due to interactions with their teacher and with each other.
For similar reasons, DB labs are also fun for teachers.
my experience: I like DB labs a lot, due to the discussions and because I can spend less time on a boring, unpleasant task (grading lab books) and more time on a productive activity (preparing for labs) that is intellectually stimulating and enjoyable. And the teaching is more satisfying because it is easy to give students immediate, customized, detailed feedback. / When I was a Teaching Assistant (TA) in a physics course with a policy of "no lab grading," during our lab-discussions I could focus my attention totally on teaching rather than judging, and students could focus on learning rather than being judged. For example, I could ask and answer any question freely, thinking only about what was best for the students. When I decided to withhold information my only motivation was pedagogical, and the purpose was to challenge students, to make them think, to let them play a more active role in their own learning. I never had to worry about whether I was "giving away too much information" about a question that later would be used to assign grades. It was a very freeing experience for me.
DB labs are an effective way to provide
frequent reflection activities for students. But
the quality of students' experience in a DB lab depends on interactions
with their Teaching Assistant, so what happens when
they get a TA with less ability, experience, or motivation?
TAs who
are socially fluent will have a great time with DB, and so will their
students.
For TAs who are
shy the structure of DB makes interactions easier because, instead
of wondering how to achieve a balance between ignoring students and bothering
them with too much attention, students initiate conversations so they can
get Xs in the grid. Although these discussions are focused on ideas
(chemistry concepts and thinking skills), this often leads to small-talk
that produces social bonding, both student-TA and student-student.
Yes, foreign TAs
who are not skilled in English will be at a disadvantage, but
1) since these TAs typically solve the "balance problem" by ignoring
students and avoiding conversations, the structure of DB will increase TA-student
interactions; 2) DB will also increase student-student interactions,
especially their discussions of ideas, and this can be intellectually stimulating
even if the TA isn't verbally fluent; 3) DB leads to increased experience
in listening and speaking; this practice will help foreign TAs improve their
English language skills, which will improve their graduate school experience
and their overall professional development.
Although consistency in TA quality is
a worthy goal, it is more important to focus on the
pragmatic question of whether "the greatest good for the greatest number
(including both students and TAs)" is promoted by discussion-based
labs.
3. How can we help students gain experience
that is more enjoyable and educationally productive?
Very few educators will dispute the main
claim of Section 2, that we should encourage students to think. This
idea is not new, and neither are the basic ideas in Section 3 or in other
sections. But designing effective labs requires careful attention
to important details, such as those examined in the remainder of this proposal.
3A. Goal-Directed Analysis of Activities
Students gain experience by doing activities. Opportunities for learning can be analyzed using an activity-and-experience grid,
student activities |
| science experiences | # 1 | # 2 | # 3 | # 4 | # 5 |
| A. generate experiments | yes | yes | |||
| B. do physical experiment | yes | yes | yes | ||
| C. hypothetico-deduction | yes | yes | yes | ||
| D. generate theories | yes | yes |
A grid shows multi-function activities (e.g., Activity
#1 promotes experiences B and C) and repeated
experiences (C occurs during 2, 3 and 5). A grid
can inspire a search for Aesop's Activities to help students learn each
type of "science experience" skill. The visual organization of information in a
grid can improve our understanding of educationally functional relationships
between activities, and can help us plan the sequencing and coordinating
(with respect to the types of experience, levels of sophistication, and
contexts) of activities within a course, to produce a mutually supportive
synergism and a more effective environment for learning.
activities/experiences
could include a discussion, experiment,
project or problem,
a question (about methods, concepts, science-and-society
issues, or...), case study (from history or
current events), computer simulation, listening or reading,....
Students can observe, collect
data, analyze data, make
and use a graph, evaluate theories using
hypothetico-deductive logic or retroductive
inference, formulate a problem, analyze an existing experiment or design
a new experiment, perform a literature search,
examine a scientific paper, solve
problems (simple or complex, algorithmic or improvisational), analyze a complex situation that involves conflicting
goal-criteria, either apply or construct concepts,
convert instructions (written or verbal) into
action, or perform cognitive/physical skills
such as filling and reading a pipet.
Activities vary in length from a mini-activity to a coherent mega-activity composed of related mini-activities.
OPTIONS.
At this point you can move to any of these sections:
3B. Strategies for Solving Problems
4. Major Challenges for Lab Education (Motivation,
Teachers, Exams)
5. The Process of Instructional Design (Cooperation, Opinions)
6. Examples of Aesop's Activities
7. My Personal Goals and
an in-depth discussion.
3B.
Strategies for Solving Problems
I have developed models of Integrated
Scientific Method (ISM) and Integrated
Design Method (IDM) that can be used to analyze instruction, or to help students
understand the relationships between the aspects of creativity and critical
thinking that are coherently combined in the problem-solving methods used
by scientists and designers.
The methods of science (in ISM) are
a special case of a more generalized method (in IDM) for designing a product,
strategy, or theory. For example, here is the way IDM describes the
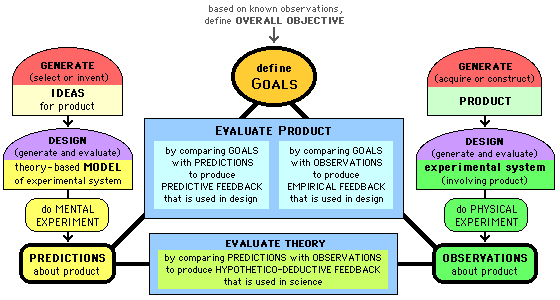
process of designing a system of labs: we first define
goals (the "learning outcome" characteristics we want for
the labs); then we develop ideas for
labs, do mental experiments (to generate predictions about outcomes) or do
actual experiments (to generate observations
of outcomes); compare predictions with goals
or compare observations with goals; adjust ideas (and maybe goals) in an effort to achieve
a match between predictions/observations and goals.
Here is a visual representation of Integrated Design Method:

4. Major Challenges for Lab Education
4A.
Student Motivation
intentional learning occurs when students invest extra mental effort, beyond what is
required to fulfill schoolwork tasks, with the intention of pursuing their
own cognitive goals.
Intentional learning is a problem-solving
approach to education because a student's goal is to
transform a current state of knowledge (and skill) into
an improved future state. This requires introspective
access to the current state of personal knowledge, foresight
to envision a useful state of improved knowledge in the future, a decision that this goal-state is desirable and worth
pursuing, a plan to transform the current state
into the goal-state, and a motivated willingness
to invest the time and effort required to reach this goal.
expectations for
transfer: Students who are not chemistry majors may be motivated
when they realize, because a teacher calls it to their attention, that similar
problem-solving methods are used in a wide range of fields (in science,
engineering,...) so they can transfer skills from chemistry to their own
field.
Intentional learning is activated when a
forward-looking student wisely asks, What can I learn now that will help
me in the future? An essential function of education is to motivate
students so they want to learn. Motivation
can be intrinsic (to enjoy an interesting activity),
extrinsic
(to perform well on an exam), and personal (to improve the long-term quality of life). Hopefully, students
will discover that thinking is fun, and they will want to do it more often
and more skillfully!
4B. Teaching Assistants
In many schools, one challenge is
to get consistently good teaching from TAs who have a wide range of ability,
experience, and motivation.
preparation: The goal is to help TAs (in weekly discussions,...) be
maximally effective with minimal investment of their time. {
an added bonus: While TAs are learning more about scientific
thinking skills, in an effort to teach these skills, this
can improve their own thinking skills, which will be useful when they do
scientific research in grad school and later in life. }
feedback: Due to their contact with students, TAs are experts on
what is happening in labs and how this can be improved.
policies: When designing labs and deciding course policies, an important
goal is to make life more pleasant and productive for TAs.
4C.
Exams to test Thinking Skills
To determine lab-grades, we can use conventional criteria
-- quality of techniques (pipeting,...), labwork (including experiments with accountability that is qualitative
and/or quantitative), discussions (or oral exams), and written reports -- plus written exams. A written lab-exam should contain a variety of questions,
including some that test higher-level thinking skills.
A set of problems to test higher-level thinking skills should:
differentiate between levels of mastery by including problems that vary in difficulty; provide an accurate measure
of thinking skills; measure the
appropriate skills -- the ones we're trying to teach.
{ i.e., there should be a high correlation between success on problems and
a mastery of the skills/knowledge being emphasized in labs }
Achieving these goals is a challenge, and we must ask
whether the "benefit per student per hour invested in making new problems
each semester" is worth the effort and cost. Why do we need new
problems? Because all questions from old exams should be available
to all students, to improve the justice in grading by eliminating the advantage
for students with access to test files from a fraternity, sorority, dormitory,
instructional center, or athletic department. If several sections
share the same labs and the same new problems, all students should take
a written lab-exam at the same time to prevent a leaking of information
from those who see the exam earlier. Also, TAs should not see the
new problems before the exam; TAs should "teach to the exam"
by knowing the general type of exam questions (similar to those on previous
exams), but if they know the specific questions there will be unavoidable
ethical dilemmas about what to teach.
comment: These criteria (differentiating between levels,...) are the usual goals in professional test design. For example, scholars constructing the American Chemical Society Exams (for the overall course, not for just labs) are trying to satisfy similar criteria, and they seem to be doing this fairly well.
5. The Process of Instructional Design
5A. Cooperation
The process of instructional design is inherently complicated,
requiring the careful consideration of many interrelated factors.
It is even more complex in a large university where ideas are coming from
course instructors and TAs, a lab director, coordinator, and support staff.
Typically there is a diversity of opinions about goals, activities, and
methods, so it is impossible to please everyone. The best solution
is to recognize the need for flexibility and cooperation, and agree that
a reasonable objective is to aim for
an optimal balancing of our alternative visions for education.
5B. Opinions
Here are a few of my opinions,
held with varying degrees of confidence:
The main goal of labs should be to help students learn thinking skills, with teaching chemistry concepts as only a secondary goal.
Students should have an opportunity
to learn in a variety of ways:
DIRECT learning
by reading or listening can be effective when techniques of actively
constructive "reception learning" are explained and emphasized,
but the most important factor is whether students
are motivated to learn.
ACTION learning
should be the main focus of labs, with students
learning by doing.
INQUIRY learning
can be very effective, especially for promoting active thinking and motivation,
but only when it is done well. Otherwise,
it will be frustrating for students and teachers. The key to effective
guided inquiry is achieving a "balance of mystery"
so a problem is not too easy or too difficult. The levels of difficulty
and activity can be adjusted by carefully controlling
the information that is provided and withheld, and by providing
scaffolding and coaching for intellectual and/or emotional support.
COOPERATIVE learning
in groups offers many valuable benefits, but sometimes (especially for experience
using lab equipment) students should work individually.
an opinion with a lower degree of
confidence:
We should consider the possibility that decreasing
the emphasis on grading may increase the quality of learning. For
example, discussion-based labs offer many educational
advantages, but a DB-grid that is totally filled with X's provides no basis
for distinguishing among students when assigning grades. /
a question: If labs are part of a general chemistry course, what are
the options for weighting the lab grades within this course? 1. less
weight than is traditional, 2. more weight than is traditional.
Discussion-based labs can be used with
any of these grading policies, but in my experience they
seem to be more compatible with 1. { Yes, I know this suggestion is
controversial, and I'm flexible about the question of course-grading policies.
}
Is there a direct relationship between
accountability and motivation? If lab grades count less when course
grades are determined, will this hinder learning in lab? Maybe.
Or maybe not, because: a. Course exams can be designed to test the
concepts and skills being learned in labs. b. We can tell students
that labs will affect their course grade more than usual if they have been
uncooperative in attitudes or actions. { During DB-labs, almost all
of my students have been consistently cooperative. } c. Internal motivation can exist even if there is no external
accountability; during labs I emphasize that, because students will
be rewarded for thinking in their professional careers (and in life as a
whole), learning how to think more skillfully has a high intrinsic value.
{ Yes, I know there are counter-arguments for each of these points. }
One option that may be very useful is
related to "a" above. We can base a large part of the lab
grade on lab exams (using in-lab "practical exams" and/or
written exams), and use DB-labs to help students prepare
for these exams.
6. EXAMPLES OF AESOP'S ACTIVITIES
Observation-Based
Thinking Skills
Experimental activities can
help students learn to use observation-based logic and to make
mental connections between levels of thinking: macro,
micro, and symbolic.
As a secondary benefit, students also learn concepts. The following
example shows how a routine procedure can become a minds-on opportunity
for learning.
not: This section has been expanded and moved to a new location, Thinking Skills in Labs - Chemistry Examples with the sections below, and more:
USING QUESTIONS TO INSPIRE ACTIVE THINKING: ...
REVERSED TRENDS: ...
CONVERTING PHYSICAL MODELS INTO MENTAL MODELS: ...
QUESTIONS ABOUT AIR:
...
AUTOMATED SUBTRACTIONS:
...
A MYSTERIOUS
TREND: ...
CONCEPTUAL PICTURES:
...
COPPER AND NITRIC
ACID: ...
BASIC SKILLS FOR DATA ANALYSIS: ...
THE PROCESS (LOGICAL AND SOCIAL) OF SCIENCE: ...
HYPOTHETICO-DEDUCTIVE
LOGIC: A handout for a homework assignment
explains the basic principles of mass spectrometry and provides mass-spec
graphs for students to analyze for practice. Then they play detectives
by using another graph to determine the structure of a C3H7Br
compound. To solve this problem, students must use HD
logic: invent two competitive theories about the structure,
use each theory to predict the corresponding graph, compare these two if-then
predictions to see where they differ, do a reality
check by observing the actual graph, compare predictions with observations,
and draw a conclusion. Surprisingly few students could finish the
entire process of HD, even after they were given an explicit step-by-step
procedure. Obviously, students need more experience with this thinking
skill that is the foundation of scientific method.
THE LOGIC
OF LE CHATELIER:
Students change the amounts of aqueous complex ions -- cobalt with water
(pink), and cobalt with chloride (blue) -- by adding NaCl or water, and
by changing the temperature. A handout, developed by myself and Jacquie
Scott (former lab director at UW), calls attention to essential ideas by
asking students to use hypothetico-deduction:
they use observations (is the color pink, blue, or an intermediate purple)
to estimate the position of equilibrium, decide how this position changes
during each step, then compare these observations with predictions based
on Le Chatelier's Principle. For the T-changes, they use retroduction
to decide whether the reaction is exothermic or endothermic. Many
concepts and skills can be learned during this lab. But without
the handout to direct their attention and promote organized active
thinking, most students would miss many of these opportunities
for learning.
CALIBRATION LOGIC: Using data I provide, students graphically
"calibrate" a new weighing scale based on readings from an old
scale that we assume is accurate. Then they do flame tests for solutions
of LiCl, Sr(NO3)2, KCl, CaBr2, and NaNO3,
and use logic to decide which chemical (assuming the cause is a single species)
produces each color. { For calcium bromide a deductive
conclusion is impossible, but a rational inductive
guess can be made. } In a second run, students do flame tests on unknown
solutions.
questions: In your detective work
on the solutions, what assumptions did you make? { Is the stockroom
telling us the truth with their bottle labels? } Does a violet
flame prove the solution contains KCl? { Could it be KBr or a substance
not contained in the known solutions? This illustrates the
asymmetry of if-then logic: "if KCl, then violet" is not
the same as "if violet, then KCl." } Could we ever
conclude with certainty that "if violet, then K"? { What
additional information is needed? Is certainty possible in science?
} If students observe a flame that is red and violet and green,
what can they conclude? { We shouldn't place restrictions on theorizing.
} Does a yellow-orange flame always indicate Na+
in a solution? { This lets us talk about false
positives and false negatives. }
Finally, students compare the two experiments:
the weighings (in two runs) and flame tests (in two runs). Between
the first and second runs of each experiment, what is constant or changing,
and what is known or unknown? What are the similarities and differences
in the logic used during the weighings and flame tests? { This lets
us discuss the usefulness and limitations of analogies. }
GUIDED INQUIRY LABS:
During guided inquiry instruction
the teacher, like a writer of a good mystery story, should aim for a level
of difficulty that is "just right" so students will not become
bored or frustrated. Ideally, students will succeed, and will feel
genuine satisfaction because in succeeding they overcame significant challenges.
The level of challenge can be adjusted
by preparation
before a problem begins (by giving students prior experience with
similar problems, by selecting the problems to be solved, and by controlling
the information that is provided and withheld) and by coaching during the process of problem solving (by observing
students, and providing guidance by asking questions, directing attention,
and promoting reflection).
A related website describes
two examples of inquiry instruction:
1) an in-depth analysis
of an innovative genetics course (from my PhD dissertation, using
a model of Integrated Scientific Method as the framework for analysis),
and
2) an outline
of a lab activity in which students design an experiment.
{ This detailed analysis of the lab will be condensed,
at some later time, and the condensed version will appear on this home-page.
}
This website describes a proposal, not a finished project.
Regarding this proposal, my professional goals are to find:
1) lively discussion and constructive feedback,
2) collaborators who want to develop creative ideas for labs, and
3) a position in a chemistry department working on instructional development,
possibly in an "educational support staff" role; my main
priority is to work with people who share my enthusiasm for the type of
education described here.
A brief resume follows.
degrees: BA
in Chemistry from Univ of California at Irvine, MS
in Chemistry from Univ of Washington, MA
in History of Science from Univ of Wisconsin (in Madison),
and PhD in Science Education (Curriculum
& Instruction) from Univ of Wisconsin.
academic awards: Was selected by
the American Chemical Society as "The Best Chemistry Student" two times, first
for all high schools of Orange County, CA, and then for U.C. Irvine.
Received NSF Fellowship for graduate study
in chemistry.
My doctoral dissertation synthesizes
ideas (mainly from scientists and philosophers, but also from sociologists,
psychologists, and historians) into a model of scientific method, and applies
this model for the integrative analysis of innovative
inquiry teaching. The main ideas are summarized in a website, Using Design Method & Scientific
Method in
Problem-Solving
Education.
For more details, see an informal "about the author" page.
The educational proposal
outlined here (and presented in a poster session
at a national meeting of the American Chemical Society, March 21, 1999)
is explored more deeply on the
main page.
all rights reserved for all material in this website,
copyright 2000 by Craig Rusbult
if you have any comments or questions, or
if you're interested in working on this project,
please contact me: craigru178@yahoo.com
craigru178@yahoo.com
the URL of this home-page is
http://www.sit.wisc.edu/~crusbult/methods/lab-99i.htm
current homepage (April 2000) for "Thinking Skills in Labs"
METHODS FOR SCIENCE AND DESIGN
LINKS TO MY PAGES ABOUT THINKING, LEARNING, AND TEACHING